As we all wait for a COVID-19 vaccine, we are getting a crash course in the FDA-approval drug approval process. If we didn’t fully appreciate it before, we are coming to understand in the global pursuit of a vaccine that massive clinical trials to prove a drug’s safety and efficacy are essential.
Surmounting this hurdle is especially important for celiac disease. Clinical drug trials are massively expensive: they are a principal reason The Tufts Center for the Study of Drug Development estimates that the average cost of developing a new FDA-approved drug is more than $2.5 billion.
Thanks to your generous support, we are deploying iCureCeliac and iQualifyCeliac, as well as other assets unique to the Foundation, to further celiac disease drug development. As you can see in this chart, the Foundation has accelerated clinical trial and study recruitment for Phase 1 and Phase 2 trials at multiple sites across the country. We are pleased to announce that we will be conducting multi-country recruitment for a worldwide clinical trial beginning this August.
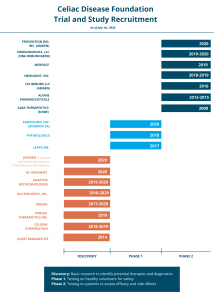
Despite the pandemic, our work is not done. We must continue to make strategic investments in both iCureCeliac and iQualifyCeliac to speed celiac disease trials. We also must continue to increase our direct investments in celiac disease research and in advocacy to compel federal investments in celiac disease researchers.
If you are able, please make a donation to the Celiac Disease Foundation today. Any gift in any amount is appreciated. Together we can defeat celiac disease.
To Our Health,
Marilyn Geller
Chief Executive