Pediatric PRO Research Report
The Celiac Disease Foundation is committed to accelerating research for celiac disease treatments and a cure. At the forefront of these efforts has been the development of essential tools that researchers and clinicians can use to lower the cost and/or increase the speed of the research and diagnostic processes. For example, our patient registry, iCureCeliac®, and our patient recruitment screening tool, iQualifyCeliac, are two research tools that we built specifically at the request of the celiac disease research community to help accelerate their work.
At the July 2021 FDA Great VI Workshop on Celiac Disease, researchers discussed the tools used in celiac disease trials to measure patient response to potential therapies, which are currently limited to findings in adults. The FDA called for the development of tools to measure pediatric response to future celiac disease therapies. In keeping with our current trajectory of pediatric resource development, which includes the NASPGHAN Clinical Guide for Pediatric Celiac Disease, the Foundation has taken the lead in developing an FDA-approved pediatric PRO (patient-reported outcome tool) that will advance research toward treatments and a cure.
The Celiac Disease Foundation is pleased to be in discussion with the University of Colorado, Denver about funding the development of this pediatric PRO. The pediatric PRO would be a huge and necessary step to accelerate research into treatment options and a cure for children with celiac disease.
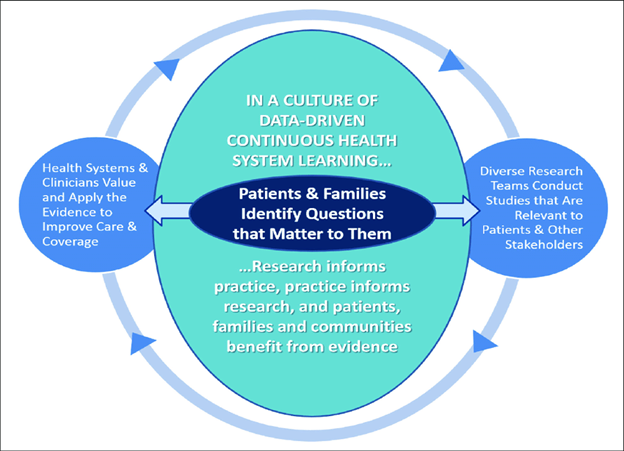
The U.S. Food and Drug Administration (FDA) defines a patient-reported outcome (PRO) as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.” PROs typically include information about health-related quality of life (HRQOL), symptoms, function, satisfaction with care or symptoms, adherence to prescribed medications or other therapy, and perceived value of treatment. The FDA advises using a PRO instrument when the concept being measured is best known by the patient or best measured from the patient’s perspective, which, as you well know, definitely includes many celiac disease symptoms. As you can see from the graphic below, PRO data are used to inform and guide patient-centered care, clinical decision-making, efficacy of clinical trials, and health policy decisions, including the approval of new therapies and cures. The pediatric PRO we are funding will support all of these objectives. I would like to highlight, however, two objectives of our investment in particular, the utility of a pediatric PRO in clinical trials and clinical decision-making.
Clinical Trials
As of this writing, there are no clinical trials for celiac disease therapies that involve individuals under the age of 18, even though we know that hundreds of thousands of children in this country suffer both physically and psychologically from celiac disease, many of who remain undiagnosed. In the coming months, the Celiac Disease Foundation will be recruiting for the first online study to capture pediatric experiences in living with celiac disease to inform how new therapies may be developed for children, as well as for adults.
Giving researchers access to an FDA-approved pediatric PRO could speed this process dramatically. Here’s how:
- The FDA requires PROs as part of the submission process for approval to begin clinical trials, to advance between trial stages, and to be approved for the market.
- PROs play a significant role as study endpoints (study outcomes that can be measured to determine potential benefit) in the development and evaluation of new therapies.
- Because some of the effects of illness are known only to patients, the FDA recommends that PRO outcomes instruments be used to assess efficacy outcomes in clinical trials.
- PROs are also used to measure the adverse effects of a therapy separately from its effectiveness.
A pediatric PRO is especially important for celiac disease, where both the range and severity of symptoms vary widely. A good PRO will help inform the risk/benefit analysis that is present in almost every drug approval decision. This is particularly relevant in celiac disease where the potential for a therapy that offers symptom relief without observable disease mitigation is a real possibility.
Clinical Decision-Making
An effective pediatric PRO instrument will not only be a valid and reliable way to collect data directly from patients to evaluate therapeutic intervention, but it will also make a positive contribution to clinical care for our children. Pediatricians and pediatric gastroenterologists can use the PRO instrument to understand symptom/disease progression at the point of care and recommend additional interventions, such as nutritional and psychological support if needed. And eventually, our goal is for the pediatric PRO to be used to determine the best therapeutics for children with celiac disease and to measure how they respond to them.
Beyond care for an individual child, data from a pediatric PRO used in clinical settings can be amassed and analyzed to improve the quality of care at a macro level, for example:
- Improving pediatric celiac diagnostic rates
- Assessing the timeliness and efficacy of follow-up serum tests and biopsies
- Advocating for insurance coverage of visits to a nutritionist
- Improving 504 accommodations and absentee policies in schools
Dr. Edwin Liu is a pediatric gastroenterologist, the Director of the Colorado Center for Celiac Disease, and holds the Taplin Endowed Chair for Celiac Disease at Children’s Hospital Colorado. He is the lead investigator on this PRO project. Dr. Liu said the following about the Foundation’s leadership in developing a pediatric PRO:
“Clinical trials often overlook the specific needs of children for a variety of reasons. We cannot assume that children are just smaller versions of adults, so it is important to remove any perceived barriers to testing in pediatrics. A pediatric PRO for celiac disease is a necessary tool for upcoming drug trials to take place in kids. We are grateful that the Foundation has not only recognized this, but has been a key driver in making this entire process happen. They have been a huge catalyst in driving policy and advancing research for the celiac community, and this is just one more example.”
Additionally, we are pleased to report that our Research Grant Award recipient, Dr. Jocelyn Silvester, Director of Research, Celiac Disease Program, Boston Children’s Hospital, and Chair of the NASPGHAN Celiac Special Interest Group, is exploring a potential new diagnostic test for celiac disease that could be an alternative to small intestinal biopsy in partnership with the University of Colorado. This test involves looking for changes in IL-2 in blood in a single-dose gluten challenge in children with celiac disease. We look forward to sharing their progress in our next report.
We are working tirelessly toward treatments and a cure for celiac disease. While we are delighted with the progress that has been made over the last several years on multiple fronts, much more is still needed. This project to create the first pediatric PRO for celiac disease is another important step in accelerating research for a cure. Please let me know if you would like to stay updated on the development of the pediatric PRO.

